Numerous germs, viruses, and carcinogens harm our health in our daily lives and work environments. If you want to live a healthy life, you should learn about different sterilization and deodorization technologies so that you may make the best decision for you and your family!
What is Photo-catalyst?
Photo-catalyst refers to photo-semiconductor materials that have a photo-catalytic function, which is exemplified by nano-sized titanium dioxide. It is coated on the surface of substrates and produces a strong catalytic degradation function under the action of ultraviolet light: it can effectively degrade toxic and harmful gases in the air; it can effectively kill a variety of bacteria, and it can decompose and harmless toxins released by bacteria or fungi; and it can also remove formaldehyde, deodorize, anti-fouling, and purify air.
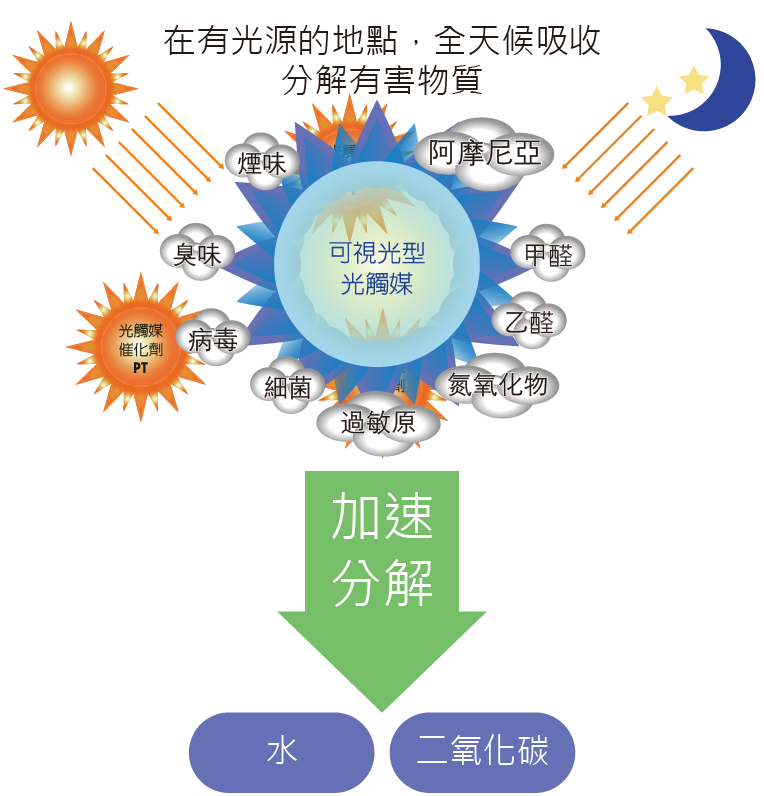
Titanium dioxide (TiO2) is employed in the field of formaldehyde removal. Simply said, the air freshener's titanium dioxide filter absorbs the formaldehyde that passes through and uses light to produce a catalytic effect, turning the formaldehyde to carbon dioxide or water. Photo-catalysts have bactericidal and virus-killing properties in addition to formaldehyde, however sufficient light is required for photo-catalyst sprays and cleaners to break down pollutants.
What is a water catalyst?
Water catalysts, unlike photocatalysts, are a brand-new technology invented by Japanese expert Dr. Daiki Miyamoto and trademarked around the world. They are sometimes known as "inorganic metal catalysts" since they are unaffected by changes in the external environment, such as light (ultraviolet rays). The mechanism's performance is determined by the "atomic group (-OH, etc.)" created by water molecules in the air, which results in a mechanism reaction. Water catalysts can degrade formaldehyde and volatile organic compounds (VOCs) in the air, and they have outstanding antibacterial and antifungal properties, as well as the ability to create a continuous antibacterial protective coating for various situations.

The water catalyst is a "composite metal complex" polymerized by high technology and is formed of alumina, silicon oxide, titanium, phosphorus, and other components. It has electronic properties due to its material composition. When applied, the solution forms a nano-scale grid-like film layer with semiconductor properties, capturing and locking volatile organic compounds (formaldehyde, acetaldehyde, benzene, toluene, xylene) released from the object and released to the film layer in the air, etc. VOCs), decomposing and converting them into H2O and CO2, while also decomposing corresponding pollutants such as microorganisms.
Decomposition principle
Water catalysts can build a conductive coating on the material's surface using oxide molecules (titanium oxide, silicon oxide) that have been dissolved in water. When this conductive coating comes into touch with airborne water, it produces oxidative free radicals (unpaired electrons). A hydroxyl group is what it's called (chemical formula is -OH). This free group (unpaired electrons) can remove the electrons from VOCs, resulting in the conversion of C (carbon), H (hydrogen), and O (oxygen) into H2O. (water). CO2 (carbon dioxide) and other non-toxic chemicals are also present.
Four characteristics
* Maintain air quality in confined spaces
* Antibacterial, deodorant, moisture-proof, formaldehyde removal
* Japan Food Analysis Center Safe Food Test, SGS Skin Sensitivity Test
* 5,000 rubbing tests on sandpaper in Japan, lasting effect and continuous decomposition for 24 hours

The water catalyst not only covers and penetrates evenly on the surface of things, exerting its performance over a vast region, but it is also difficult to fall off and remove due to its outstanding stability. Its "hydrophilicity" and "self-cleaning" properties, as well as its grid The structure of the film-like layer is such that dust and grime do not adhere to the film layer's material.
Water catalysts are non-hazardous chemicals, according to international regulations, ensuring their safety and non-toxicity. When utilized, they can come into touch with human flesh without causing harm, and they won't harm items by corroding them or changing their color, nor will they harm the environment (water, soil, etc.) by generating pollution.
Water-catalyst VS Photo-catalyst
|
Items |
Water-catalyst |
Photo-catalyst |
|
Tightness |
Adhesion is tight. After special testing in Japan, the metal coating can reach jade hardness, and the abrasive paper has abrasion resistance of 5000 times without abrasion. |
Easy to wear, low wear resistance |
|
Transparency |
Transparent and colorless after drying |
Cloudy, white powder remains after drying |
| Chemical decomposition conditions | Chemical action caused by contact with moisture in the air, continuous decomposition all weather | Chemical action only when sunlight reaches a certain intensity |
|
Application scope |
Outdoor, non-vacuum all environments |
Only suitable for outdoor construction, indoor use must be additionally used with UV light |
| Deodorizing effect | Continuously decomposes offensive odors caused by bacteria and chemical molecules, and has a particularly significant effect on smoke, toilet and Armenian odor |
Can only remove part of the chemical odor, the validity period is relatively short |

Water catalysts and photo-catalysts, on the other hand, can accomplish significant effects including deodorization, antifouling, antibacterial, anti-mildew, and gas reduction. Water catalysts, on the other hand, are more effective and sustainable due to their high reliance on water and the long-term nature of regeneration. Antibacterial properties.
The water catalyst was tested in Japan and found to be efficient against bacteria including pneumoniae, Pseudomonas aeruginosa, coliform, and bacillus cereus, as well as being safe to contact human skin. It may transform toxic compounds like VOCs into safe substances like water and carbon dioxide as long as it is exposed to moisture in the air.
Water catalysts have a longer effect than photocatalysts since they do not require light to induce breakdown.



